PS1.B: Chemical Reactions
In this video Paul Andersen explains how chemical reactions progress as bonds are broken and reformed reformed. He explains the difference between changes in state and changes in molecules. He discussed collision theory and explains why increases in temperature and concentration can increase reaction rates. He also discusses the conservation of matter in chemical reactions. The video also contains a teaching progression for chemical reactions from grades K-12.
PS1.A: Structure and Properties of Matter
In the first physical science video for the Next Generation Science Standards Paul Andersen explains the structure and properties of matter. He starts by explaining how all matter is made of about 100 smaller particles called matter. He explains a teaching progression for introducing the topic of matter K-12. This begins with a brief introduction to substances that can be scientifically observed at many levels. This eventually builds through molecules and pure substances to the subatomic structure of atoms and the importance of binding energy.
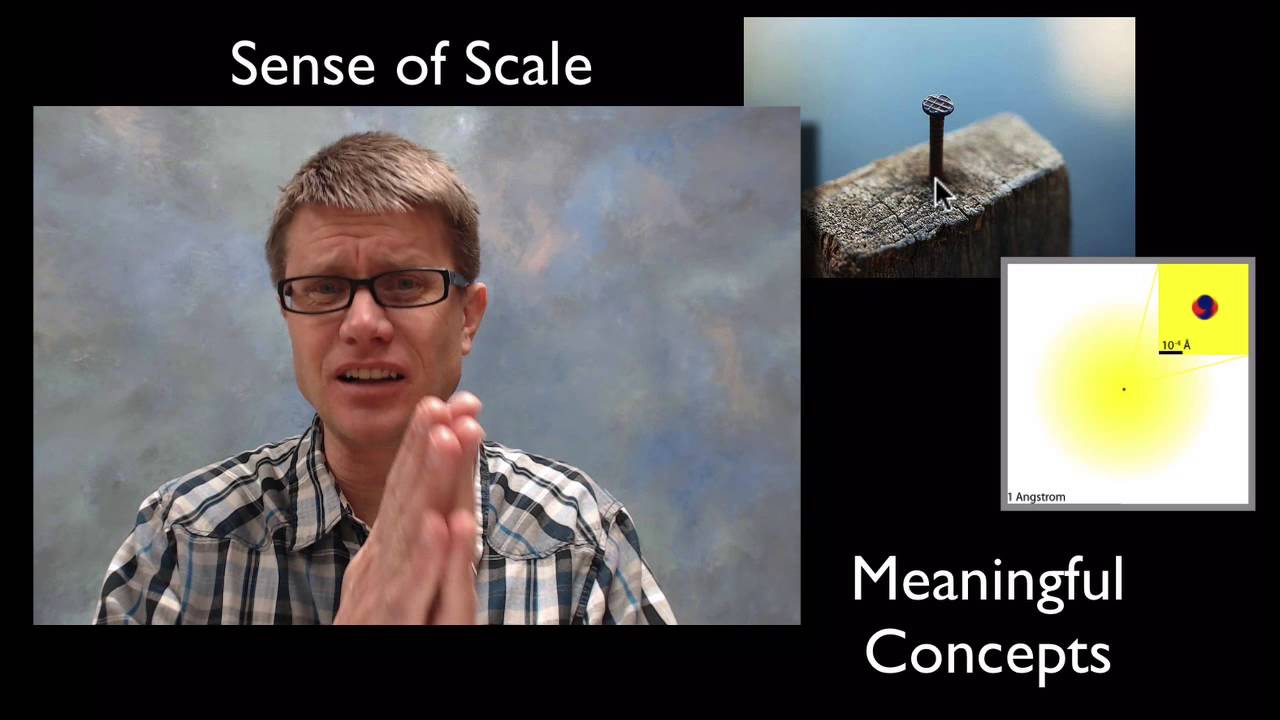
CCC3: Scale, Proportion, and Quantity
In this video Paul Andersen explains the importance of scale in science and engineering. The Universe varies in size along three scales: size, timespan, and energy. Many phenomenon are too small and fast, or two large and slow to observe. We use the tools of proportion and units of measure to comprehend different scales. The video ends with a progression of instruction from K-12
SEP5: Using Mathematics and Computational Thinking
Paul Andersen explains how mathematics and computational thinking can be used by scientists to represent variables and by engineers to improve design. He starts by explaining how mathematics is at the root of all sciences. He then defines computational thinking and gives you a specific example of computational modeling. He finishes the video with a teaching progression for this practice.