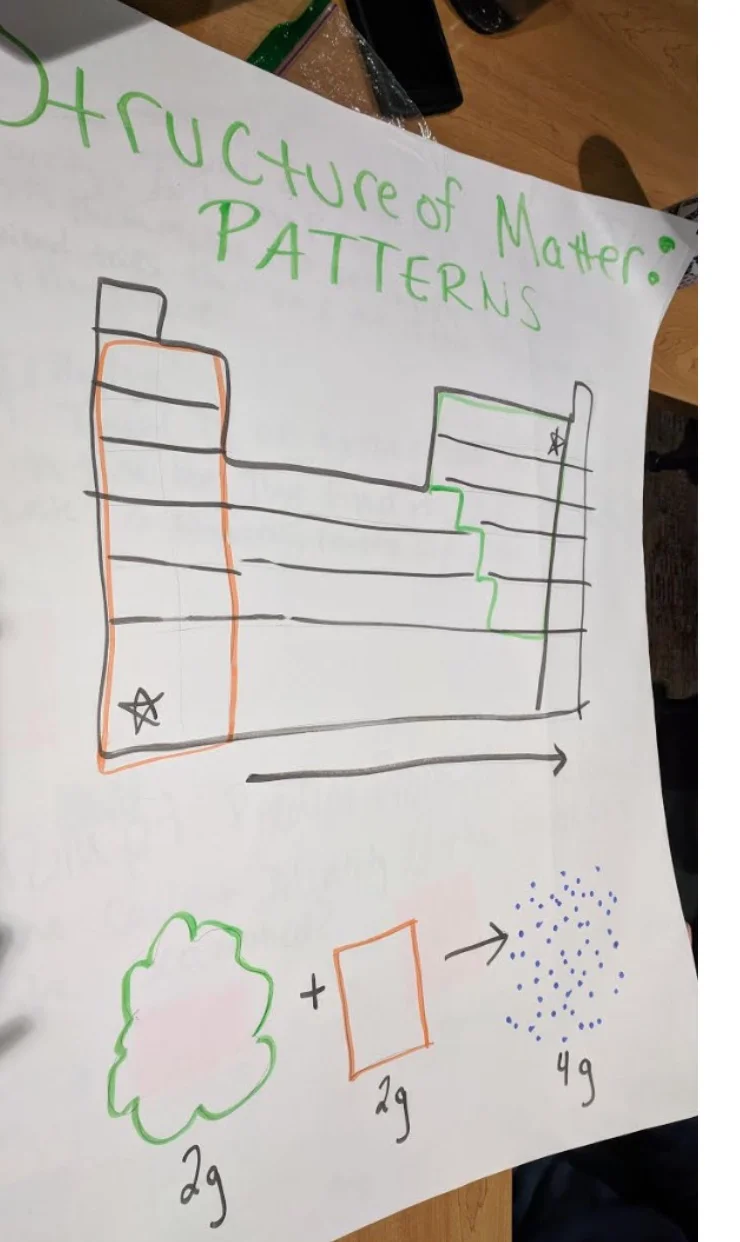
HS-PS1-1: Valence Electrons and Properties of Elements
Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy level of atoms.
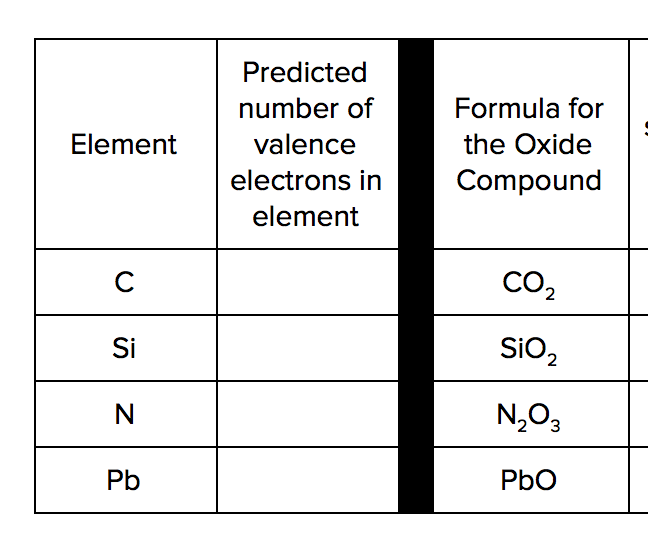
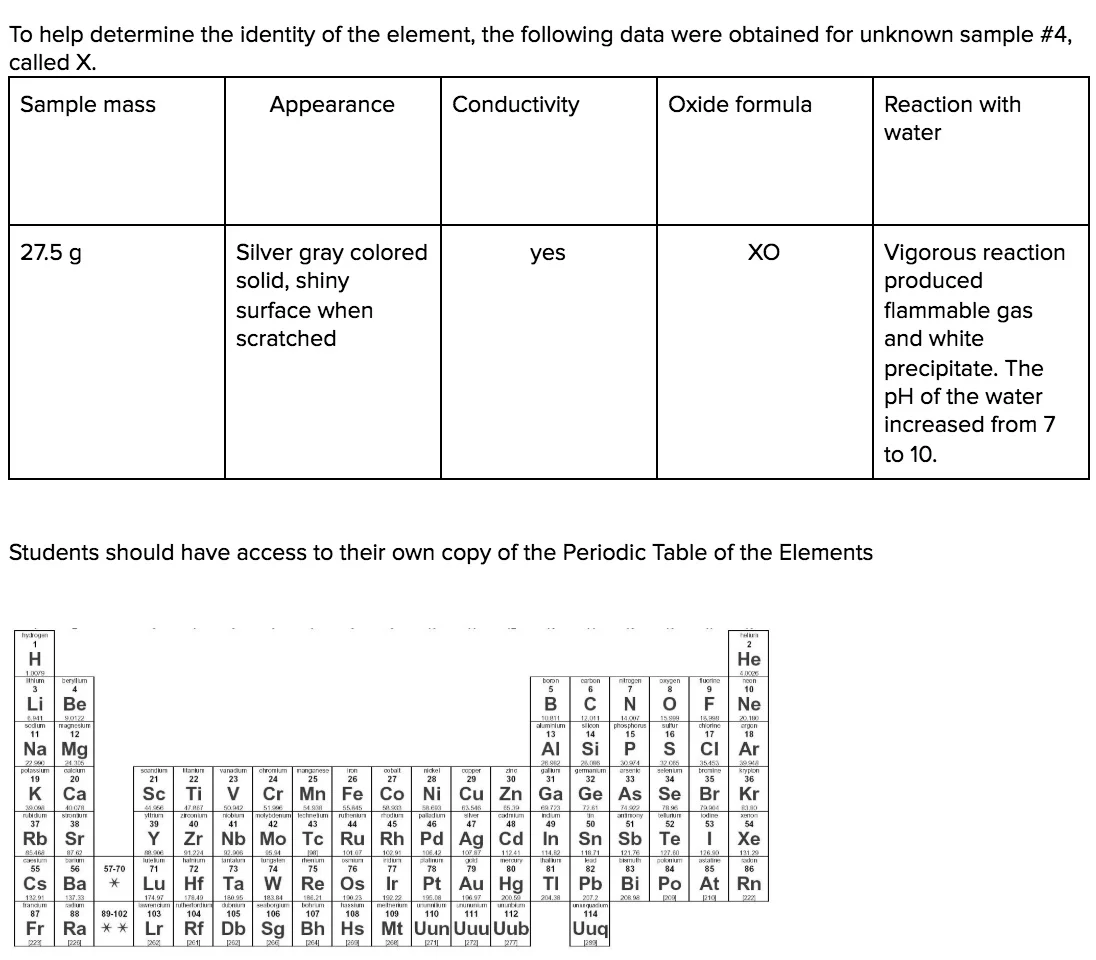
Clarification Statement: Examples of properties that could be predicted from patterns could include reactivity of metals, types of bonds formed, numbers of bonds formed, and reactions with oxygen.
Assessment Boundary: Assessment is limited to main group elements. Assessment does not include quantitative understanding of ionization energy beyond relative trends.
Science Practices
Developing and Using Models
Disciplinary Core Ideas
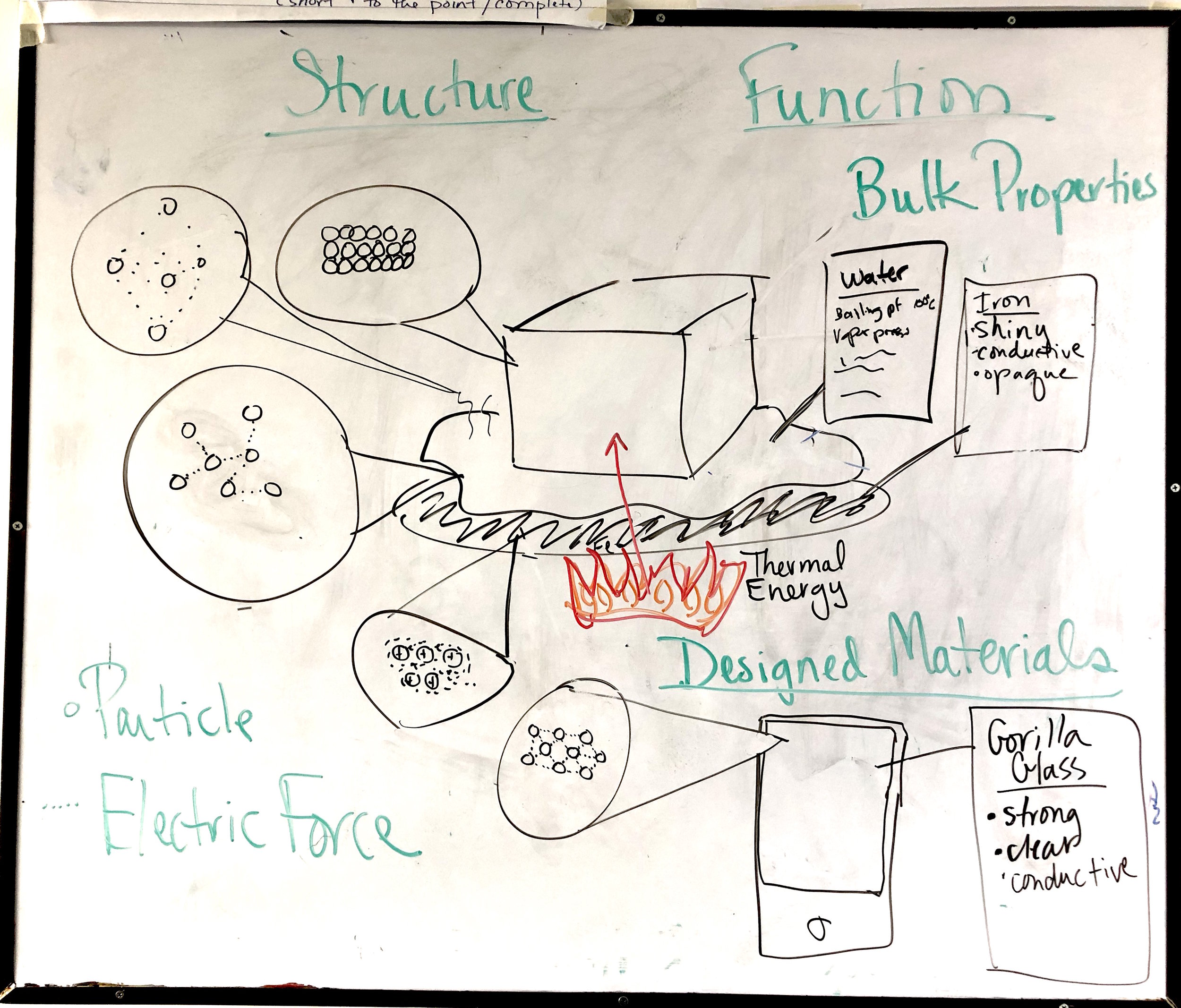
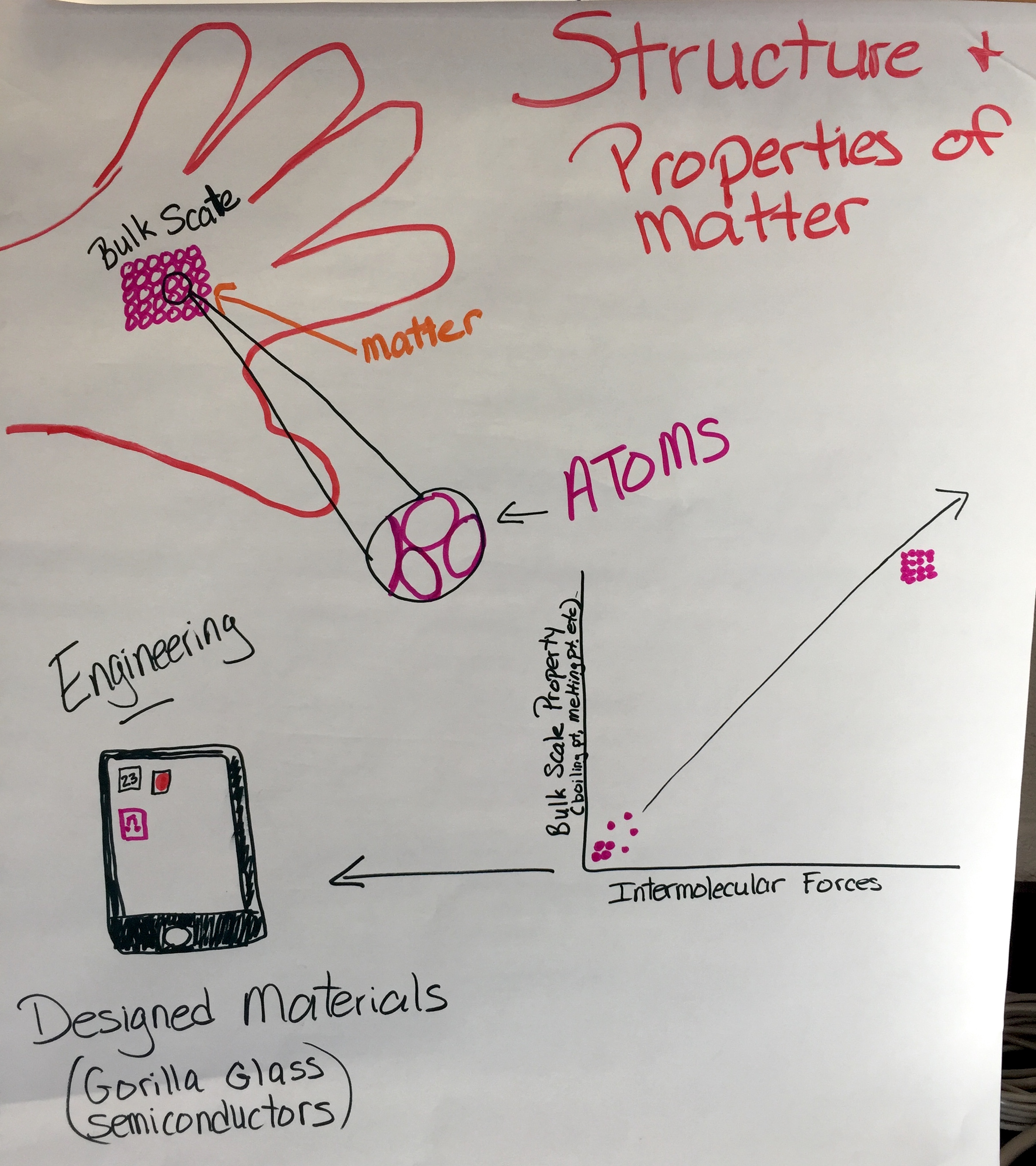
PS1.A: Structure and Properties of Matter
Crosscutting Concepts
Patterns
Assessments
The Wonder of Science Assessments
Shared Assessments
The following assessments were shared by teachers implementing the NGSS. Many of these are drafts and should be used accordingly. Feel free to improve these assessments or contribute your own. Learn more here.
Instructional Resources
Mini Lessons
The Wonder of Science Resources
Anchor Charts
Phenomena
Videos
Learning Plans
Storylines
Common Core Connections
ELA/Literacy
RST.9-10.7 - Translate quantitative or technical information expressed in words in a text into visual form (e.g., a table or chart) and translate information expressed visually or mathematically (e.g., in an equation) into words.
*Next Generation Science Standards is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of this product, and do not endorse it. Visit the official NGSS website.