HS-PS1-2: Simple Chemical Reactions
Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
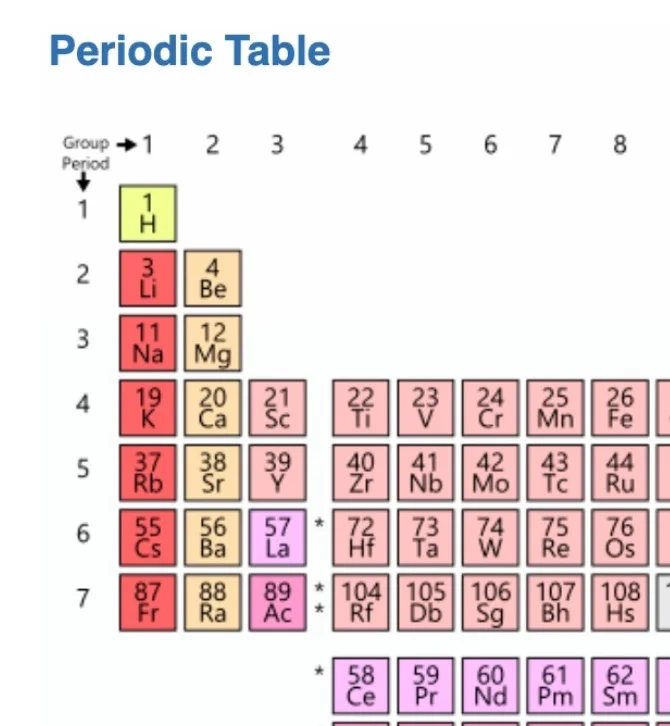
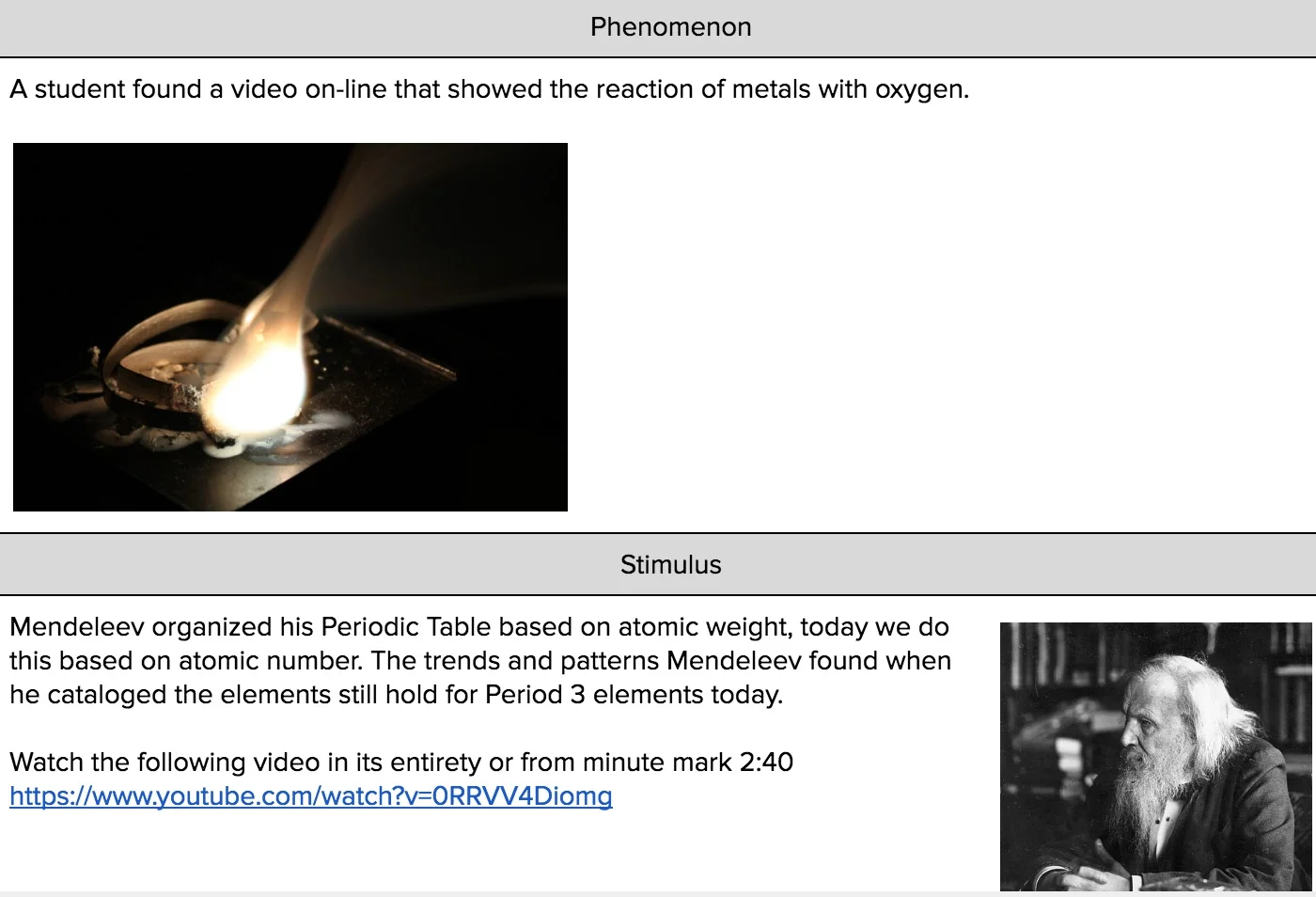
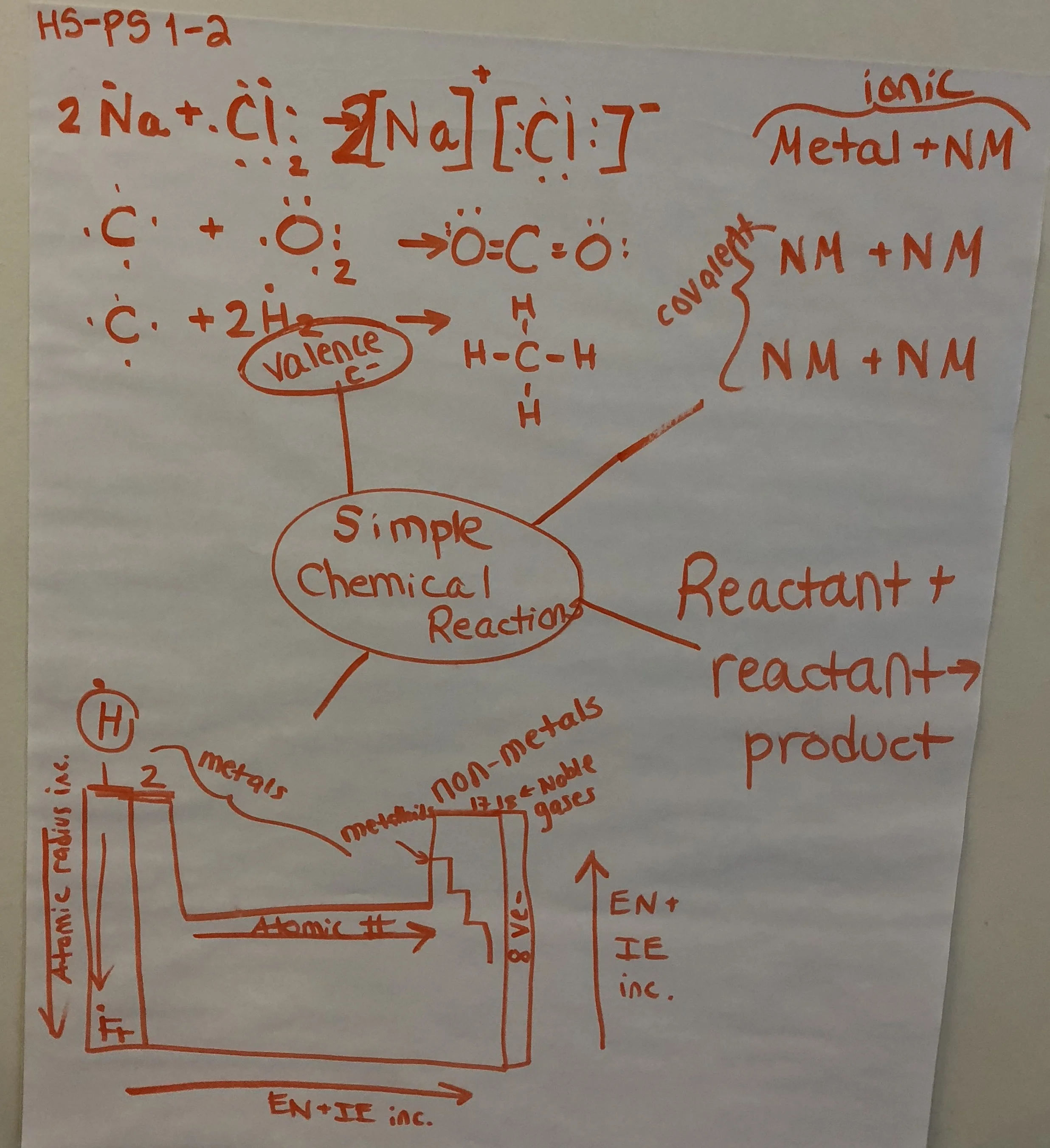
Clarification Statement: Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.
Assessment Boundary: Assessment is limited to chemical reactions involving main group elements and combustion reactions.
Science Practices
Constructing Explanations
Disciplinary Core Ideas
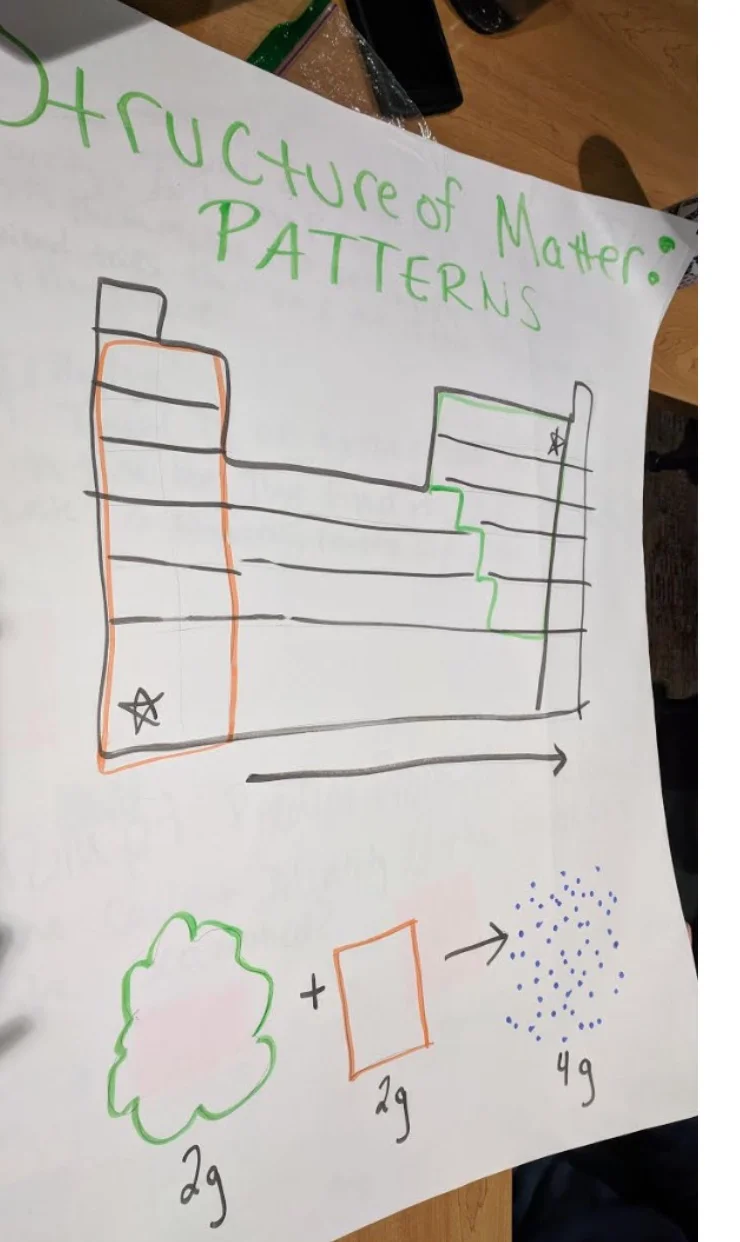
PS1.A: Structure and Properties of Matter
PS1.B: Chemical Reactions
Crosscutting Concepts
Patterns
Assessments
The Wonder of Science Assessments
Shared Assessments
The following assessments were shared by teachers implementing the NGSS. Many of these are drafts and should be used accordingly. Feel free to improve these assessments or contribute your own. Learn more here.
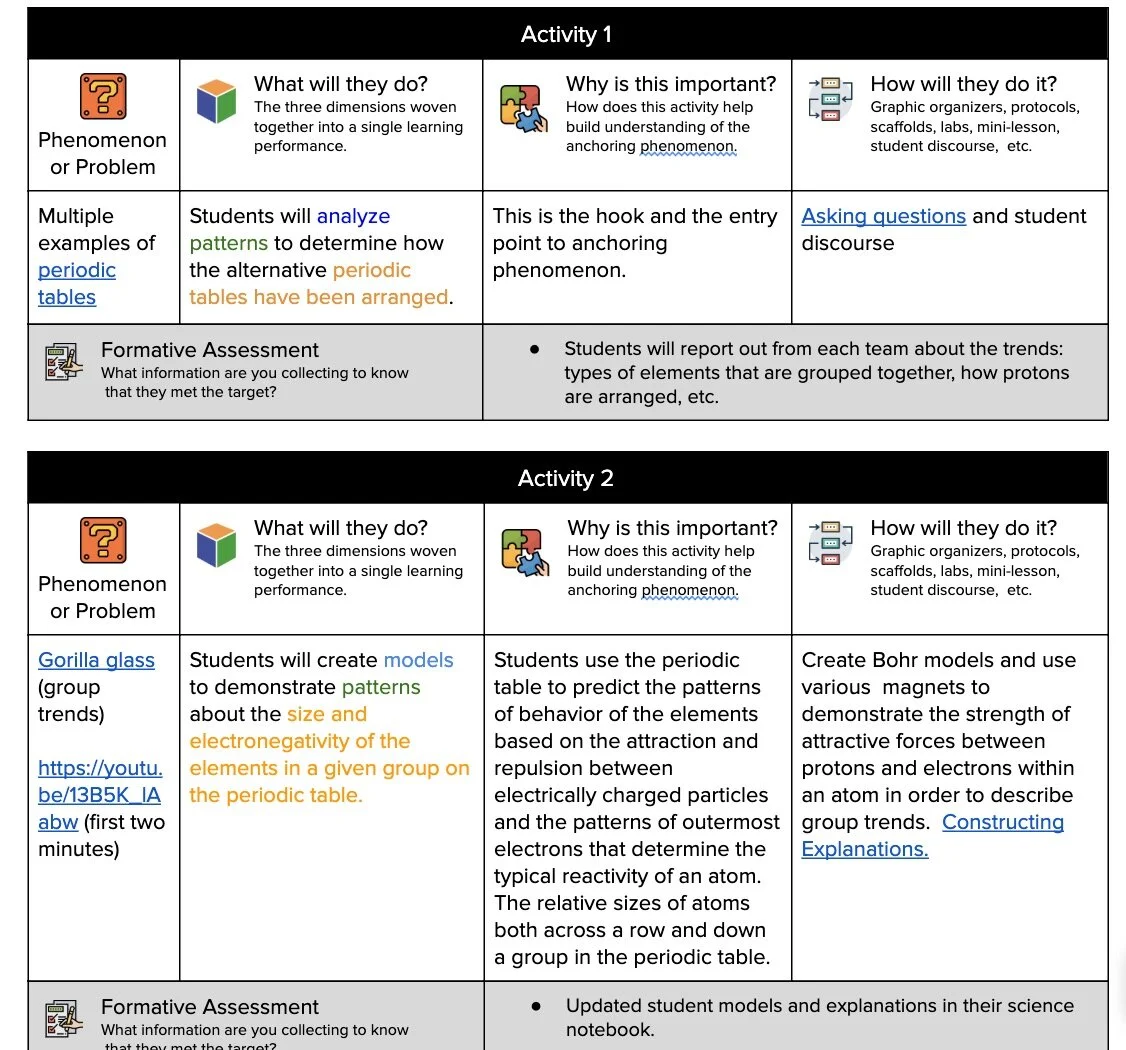
Instructional Resources
Mini Lessons
The Wonder of Science Resources
Anchor Charts
Phenomena
Videos
Learning Plans
Storylines
Common Core Connections
ELA/Literacy
WHST.9-12.2 - Write informative/explanatory texts, including the narration of historical events, scientific procedures/ experiments, or technical processes.
WHST.9-12.5 - Develop and strengthen writing as needed by planning, revising, editing, rewriting, or trying a new approach, focusing on addressing what is most significant for a specific purpose and audience.
Mathematics
HSN-Q.A.1 - Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
HSN-Q.A.3 - Choose a level of accuracy appropriate to limitations on measurement when reporting quantities.
*Next Generation Science Standards is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of this product, and do not endorse it. Visit the official NGSS website.