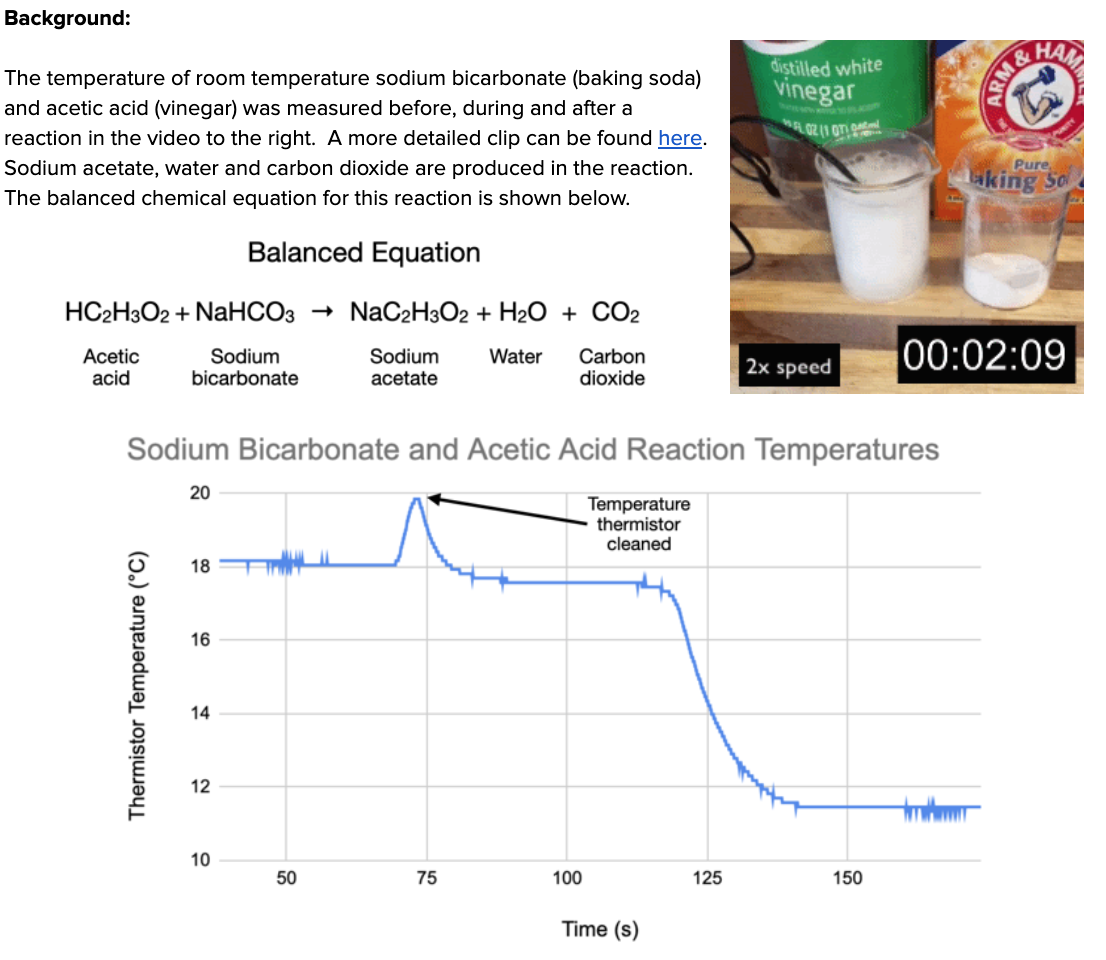
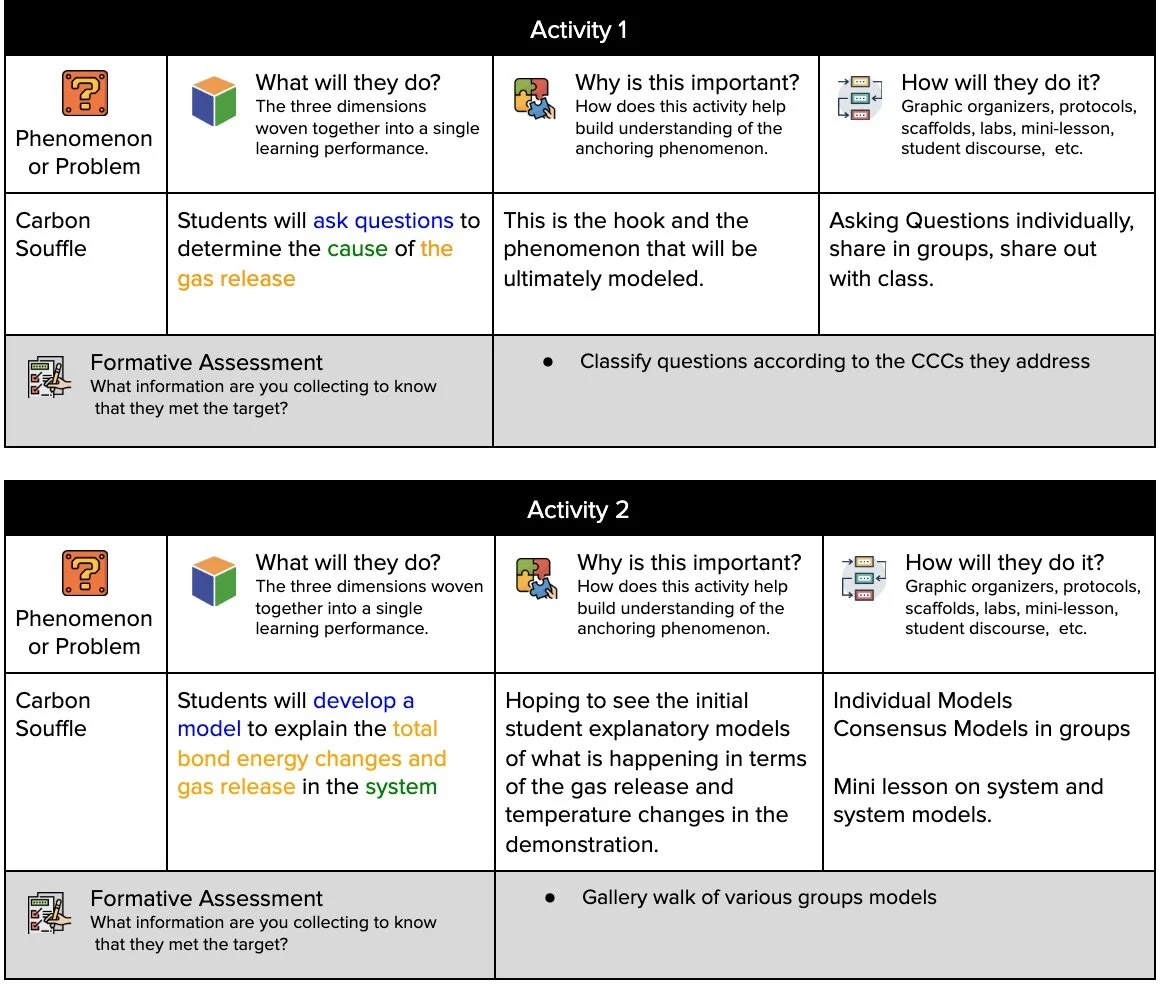
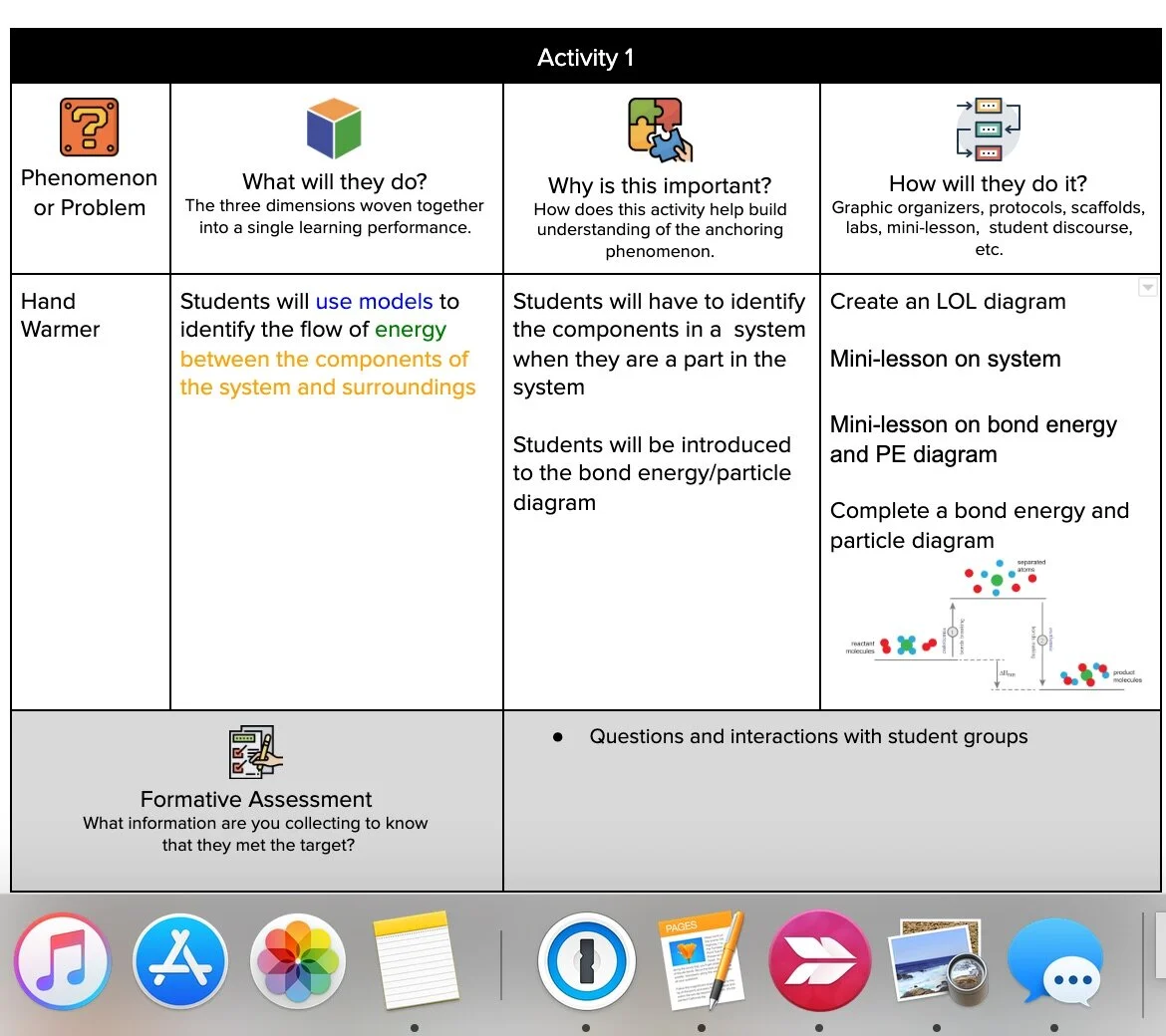
HS-PS1-4: Total Bond Energy Change in Chemical Reactions
Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy. (Energy and Matter)
Clarification Statement: Emphasis is on the idea that a chemical reaction is a system that affects the energy change. Examples of models could include molecular-level drawings and diagrams of reactions, graphs showing the relative energies of reactants and products, and representations showing energy is conserved.
Assessment Boundary: Assessment does not include calculating the total bond energy changes during a chemical reaction from the bond energies of reactants and products.
Science Practices
Developing and Using Models
Disciplinary Core Ideas
PS1.A: Structure and Properties of Matter
PS1.B: Chemical Reactions
Crosscutting Concepts
Energy and Matter
Assessments
The Wonder of Science Assessments
Shared Assessments
The following assessments were shared by teachers implementing the NGSS. Many of these are drafts and should be used accordingly. Feel free to improve these assessments or contribute your own. Learn more here.
Instructional Resources
Mini Lessons
The Wonder of Science Resources
Anchor Charts
Phenomena
Videos
Learning Plans
Storylines
Common Core Connections
ELA/Literacy
SL.11-12.5 - Make strategic use of digital media (e.g., textual, graphical, audio, visual, and interactive elements) in presentations to enhance understanding of findings, reasoning, and evidence and to add interest.
Mathematics
HSN-Q.A.1 - Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
HSN-Q.A.2 - Define appropriate quantities for the purpose of descriptive modeling.
HSN-Q.A.3 - Choose a level of accuracy appropriate to limitations on measurement when reporting quantities.
MP.4 - Model with mathematics.
*Next Generation Science Standards is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of this product, and do not endorse it. Visit the official NGSS website.