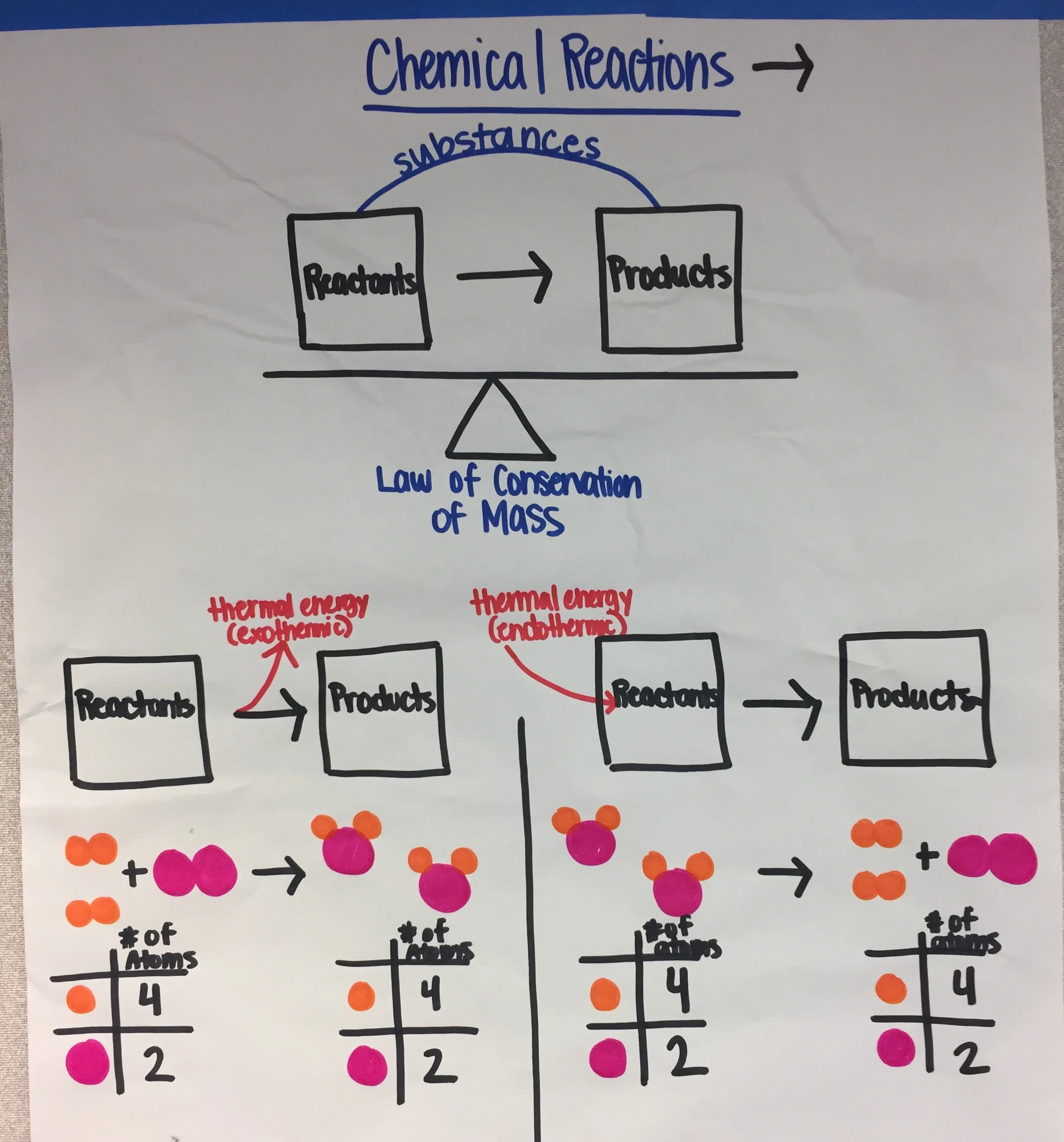
MS-PS1-5: Conservation of Atoms in Reactions
Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved. (Energy and Matter)
Clarification Statement: Emphasis is on law of conservation of matter and on physical models or drawings, including digital forms, that represent atoms.
Assessment Boundary: Assessment does not include the use of atomic masses, balancing symbolic equations, or intermolecular forces.
Science Practices
Developing and Using Models
Disciplinary Core Ideas
PS1.B: Chemical Reactions
Crosscutting Concepts
Energy and Matter
Assessments
The Wonder of Science Assessments
Shared Assessments
The following assessments were shared by teachers implementing the NGSS. Many of these are drafts and should be used accordingly. Feel free to improve these assessments or contribute your own. Learn more here.
Instructional Resources
Mini Lessons
The Wonder of Science Resources
Anchor Charts
Phenomena
Videos
Learning Plans
Storylines
Common Core Connections
ELA/Literacy
RST.6-8.7 - Integrate quantitative or technical information expressed in words in a text with a version of that information expressed visually (e.g., in a flowchart, diagram, model, graph, or table).
Mathematics
6.RP.A.3 - Use ratio and rate reasoning to solve real-world and mathematical problems, e.g., by reasoning about tables of equivalent ratios, tape diagrams, double number line diagrams, or equations.
MP.2 - Reason abstractly and quantitatively.
MP.4 - Model with mathematics.
*Next Generation Science Standards is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of this product, and do not endorse it. Visit the official NGSS website.