HS-PS1-3: Electrical Forces and Bulk Scale Structure
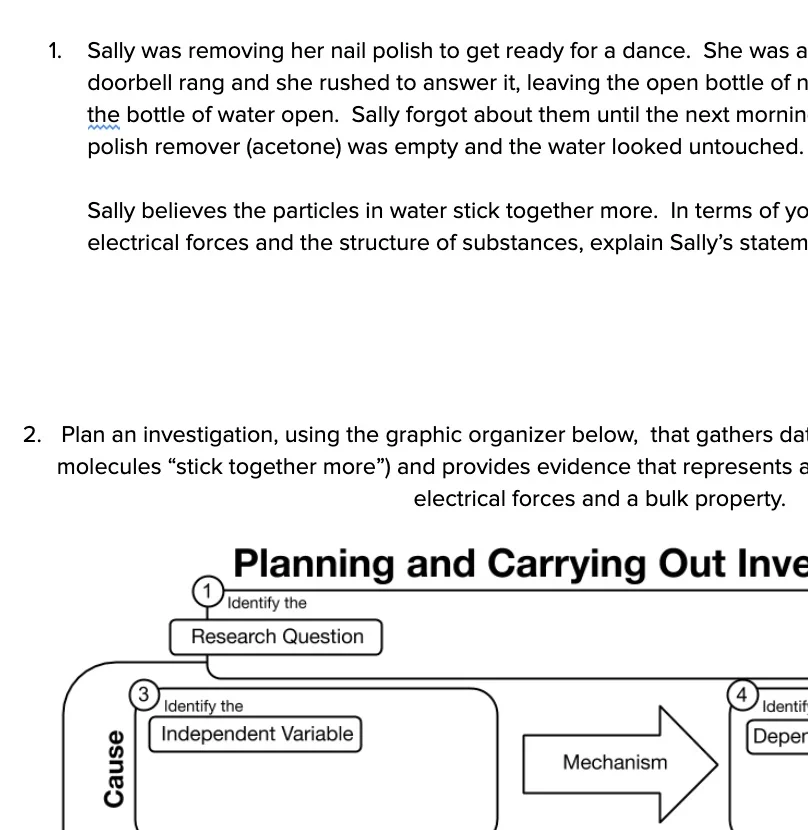
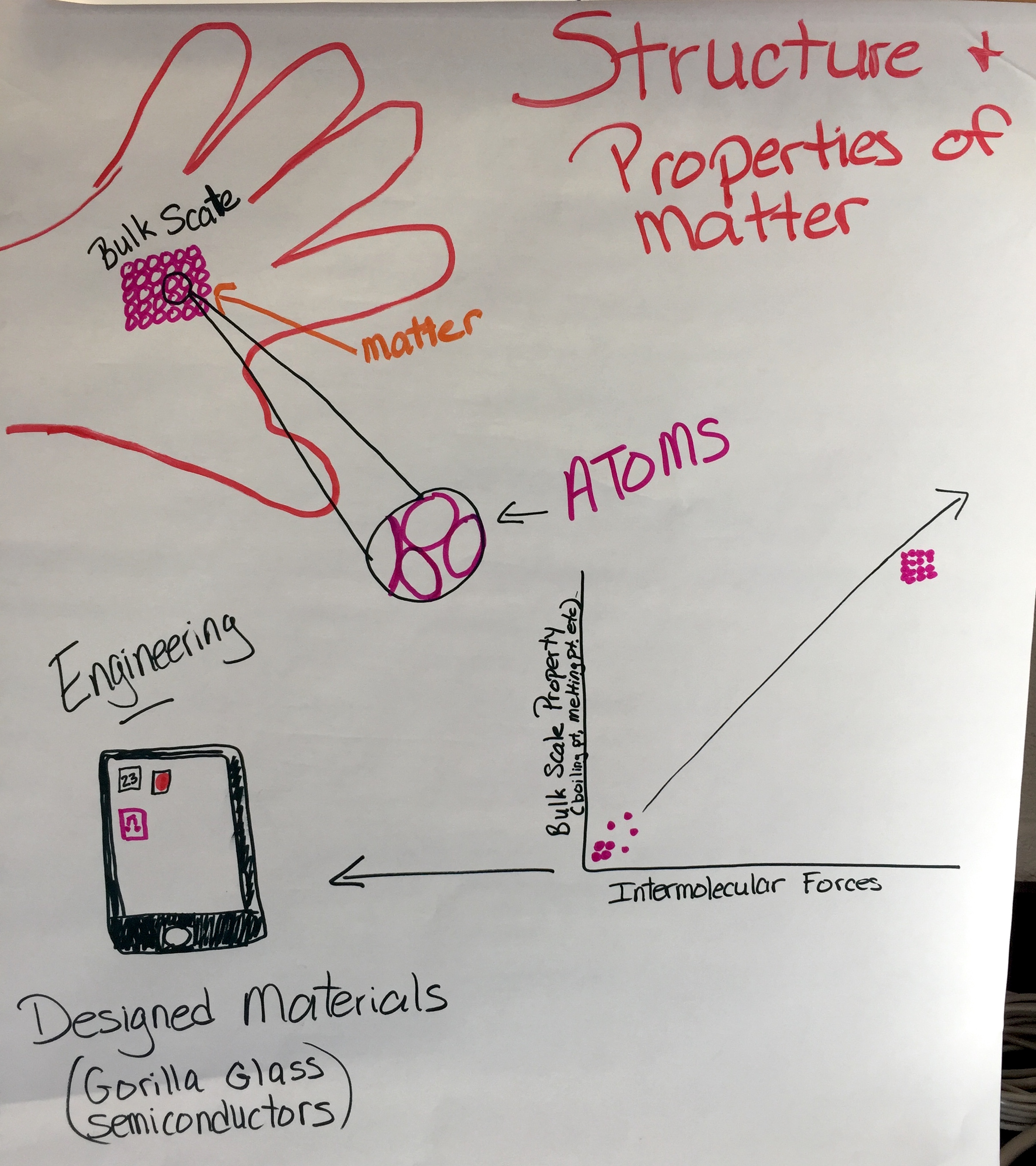
Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles. (Patterns)
Clarification Statement: Emphasis is on understanding the strengths of forces between particles, not on naming specific intermolecular forces (such as dipole-dipole). Examples of particles could include ions, atoms, molecules, and networked materials (such as graphite). Examples of bulk properties of substances could include the melting point and boiling point, vapor pressure, and surface tension.
Assessment Boundary: Assessment does not include Raoult’s law calculations of vapor pressure.
Science Practices
Planning and Carrying Out Investigations
Disciplinary Core Ideas
PS1.A: Structure and Properties of Matter
PS2.B: Types of Interactions
Crosscutting Concepts
Patterns
Assessments
The Wonder of Science Assessments
Shared Assessments
The following assessments were shared by teachers implementing the NGSS. Many of these are drafts and should be used accordingly. Feel free to improve these assessments or contribute your own. Learn more here.
Instructional Resources
Mini Lessons
The Wonder of Science Resources
Anchor Charts
Phenomena
Videos
Learning Plans
Storylines
Common Core Connections
ELA/Literacy
RST.11-12.1 - Cite specific textual evidence to support analysis of science and technical texts, attending to important distinctions the author makes and to any gaps or inconsistencies in the account.
WHST.11-12.7 - Conduct short as well as more sustained research projects to answer a question (including a self-generated question) or solve a problem; narrow or broaden the inquiry when appropriate; synthesize multiple sources on the subject, demonstrating understanding of the subject under investigation.
WHST.11-12.8 - Gather relevant information from multiple authoritative print and digital sources, using advanced searches effectively; assess the strengths and limitations of each source in terms of the specific task, purpose, and audience; integrate information into the text selectively to maintain the flow of ideas, avoiding plagiarism and over reliance on any one source and following a standard format for citation.
WHST.11-12.9 - Draw evidence from informational texts to support analysis, reflection, and research.
Mathematics
HSN-Q.A.1 - Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
HSN-Q.A.3 - Choose a level of accuracy appropriate to limitations on measurement when reporting quantities.
*Next Generation Science Standards is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of this product, and do not endorse it. Visit the official NGSS website.