MS-PS1-4: Thermal Energy and Particle Motion
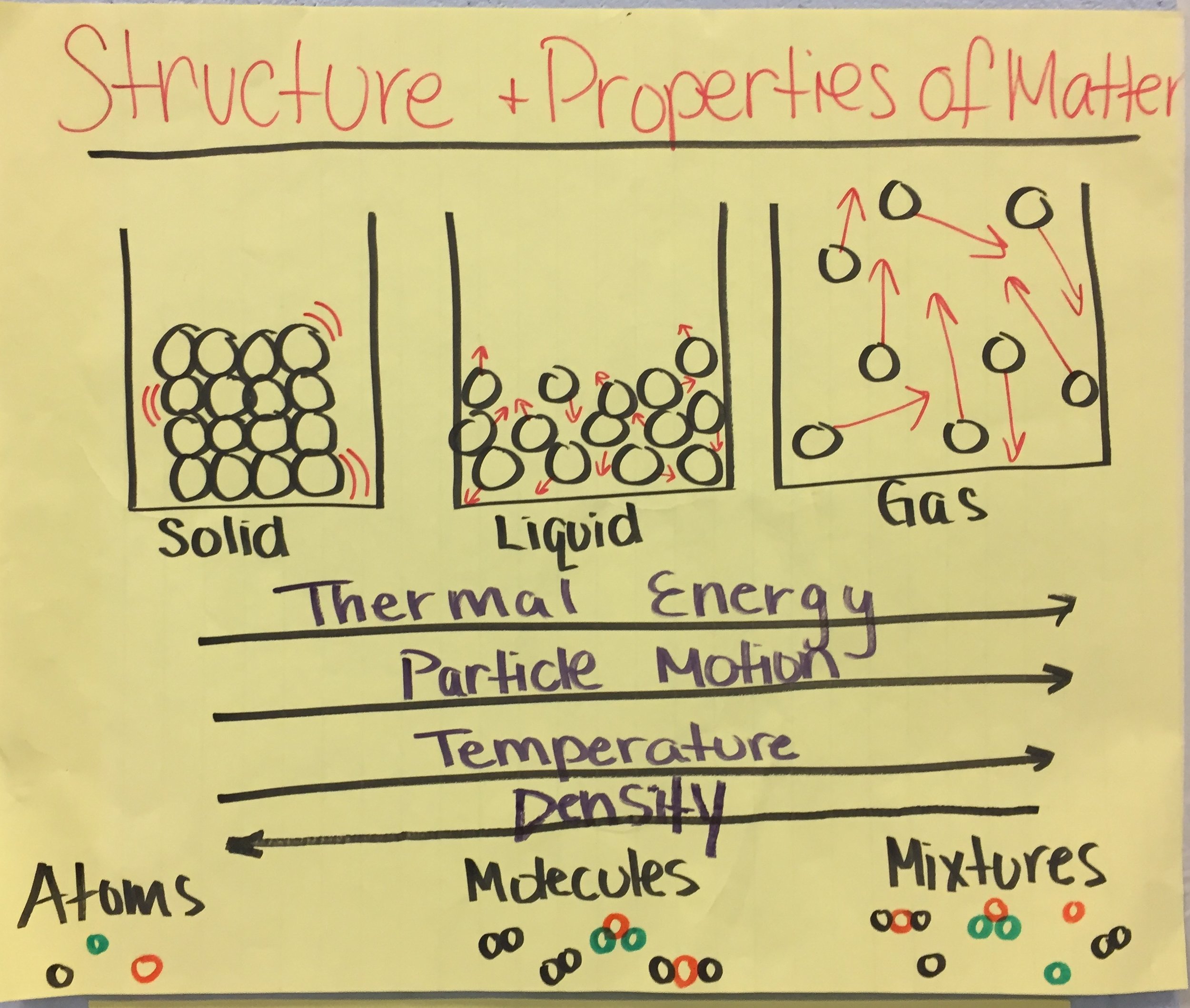
Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed. (Cause and Effect)
Clarification Statement: Emphasis is on qualitative molecular-level models of solids, liquids, and gases to show that adding or removing thermal energy increases or decreases kinetic energy of the particles until a change of state occurs. Examples of models could include drawing and diagrams. Examples of particles could include molecules or inert atoms. Examples of pure substances could include water, carbon dioxide, and helium.
Assessment Boundary: none
Science Practices
Developing and Using Models
Disciplinary Core Ideas
PS1.A: Structure and Properties of Matter
PS3.A: Definitions of Energy
Crosscutting Concepts
Cause and Effect
Assessments
The Wonder of Science Assessments
Shared Assessments
The following assessments were shared by teachers implementing the NGSS. Many of these are drafts and should be used accordingly. Feel free to improve these assessments or contribute your own. Learn more here.
Instructional Resources
Mini Lessons
The Wonder of Science Resources
Anchor Charts
Phenomena
Video
Learning Plans
Storylines
Common Core Connections
ELA/Literacy
RST.6-8.7 - Integrate quantitative or technical information expressed in words in a text with a version of that information expressed visually (e.g., in a flowchart, diagram, model, graph, or table).
Mathematics
6.NS.C.5 - Understand that positive and negative numbers are used together to describe quantities having opposite directions or values (e.g., temperature above/below zero, elevation above/below sea level, credits/debits, positive/negative electric charge); use positive and negative numbers to represent quantities in real-world contexts, explaining the meaning of 0 in each situation.
*Next Generation Science Standards is a registered trademark of Achieve. Neither Achieve nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of this product, and do not endorse it. Visit the official NGSS website.